The statement that’s NOT true as regards equilibrium in the question is ( The concentrations of reactants and products stop changing) Chemical equilibrium can be regarded as a condition in the course of a reversible chemical reaction whereby there is no net change as regards the the amounts of reactants as well as products that occurs.; A reversible chemical reaction can be regarded as a
Mastering Vectors and Equilibrium – Physics XI
Chemistry Question Which of the following conditions is always true at equilibrium? i. ΔG° = ΔG° ^ 1 1 ii. Q = 0 iii. K = 1 iv. ΔG = 0 Solution Answered 2 months ago Create a free account to view solutions By signing up, you accept Quizlet’s Terms of Service and Privacy Policy Continue with Google Continue with Facebook

Source Image: predragrajsic.blogspot.com
Download Image
20 questions 1. Multiple Choice 1 minute 1 pt Given the equation representing a reaction at equilibrium: N2(g) + 3H2(g) –> 2NH3(g) + energy Which change causes the equilibrium to shift to the right? decreasing the concentration of H2(g) decreasing the pressure increasing the concentration of N2(g) increasing the temperature 2. Multiple Choice

Source Image: homework.study.com
Download Image
Solved Which of the following is/are true concerning | Chegg.com Chemistry Law of Mass Action Question Which of the following is not true for the equilibrium reaction? N 2(g)+O2(g) ⇌ 2N O(g); ΔH = 180kJ −1 A The formation of NO is increased at higher temperature B The volume change at constant pressure does not affect the equilibrium C The pressure change at constant volume does not affect the equilibrium D

Source Image: www.slideshare.net
Download Image
Which Of The Following Is Not True Of Equilibrium
Chemistry Law of Mass Action Question Which of the following is not true for the equilibrium reaction? N 2(g)+O2(g) ⇌ 2N O(g); ΔH = 180kJ −1 A The formation of NO is increased at higher temperature B The volume change at constant pressure does not affect the equilibrium C The pressure change at constant volume does not affect the equilibrium D Oct 9, 2022When an object is in equilibrium, it means that said object is not accelerating. Since the problem mentions both rotational and translational motion, we need to look at both rotational and translational acceleration. An object can be in equilibrium if a = 0 m/s 2 AND α = 0 rad/s 2. Note that the acceleration says nothing about any velocity the
Market Equilibrium Analysis | PPT
Learn Test Match Q-Chat Get a hint A system at a state of chemical equilibrium is Click the card to flip 👆 a: microscopically dynamic and macroscopically static – forward and reverse reactions are dynamic → microscopic – changes in quantities of substances are static (motionless) → macroscopic Click the card to flip 👆 1 / 13 1 / 13 Flashcards Learn This history of browser engines (1990-2020) shows why we must keep Gecko alive. : r/firefox

Source Image: www.reddit.com
Download Image
Solved Of the following, which is not true of a system at | Chegg.com Learn Test Match Q-Chat Get a hint A system at a state of chemical equilibrium is Click the card to flip 👆 a: microscopically dynamic and macroscopically static – forward and reverse reactions are dynamic → microscopic – changes in quantities of substances are static (motionless) → macroscopic Click the card to flip 👆 1 / 13 1 / 13 Flashcards Learn

Source Image: www.chegg.com
Download Image
Mastering Vectors and Equilibrium – Physics XI The statement that’s NOT true as regards equilibrium in the question is ( The concentrations of reactants and products stop changing) Chemical equilibrium can be regarded as a condition in the course of a reversible chemical reaction whereby there is no net change as regards the the amounts of reactants as well as products that occurs.; A reversible chemical reaction can be regarded as a

Source Image: www.pinterest.com
Download Image
Solved Which of the following is/are true concerning | Chegg.com 20 questions 1. Multiple Choice 1 minute 1 pt Given the equation representing a reaction at equilibrium: N2(g) + 3H2(g) –> 2NH3(g) + energy Which change causes the equilibrium to shift to the right? decreasing the concentration of H2(g) decreasing the pressure increasing the concentration of N2(g) increasing the temperature 2. Multiple Choice

Source Image: www.chegg.com
Download Image
Feedback mechanism – Definition and Examples – Biology Online Dictionary Solution Verified by Toppr Correct option is D) solution: option a: It’s an endothermic reaction as ΔH is positive as temperature increases equilibrium will try to balnce it out by moving right side so its true option b : on increasing pressure there is no change in equilibrium as Δn=0 option C :
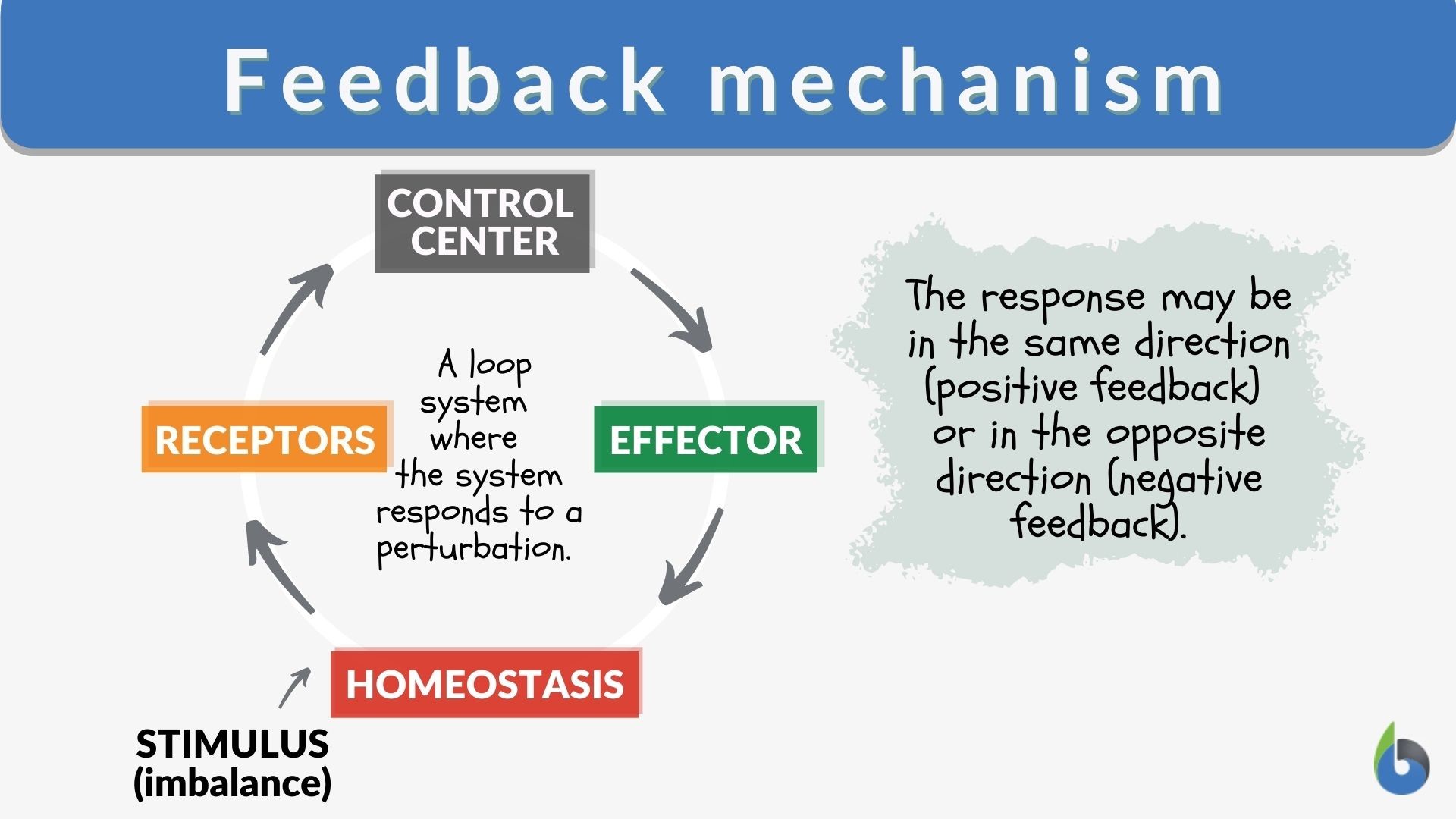
Source Image: www.biologyonline.com
Download Image
Disha Commerce Classes. – 👋Hi Everyone, Lets begin a day with a very positive and motivating quote 🔥 ” Always remember you are a braver than you believe, stronger than you seem Chemistry Law of Mass Action Question Which of the following is not true for the equilibrium reaction? N 2(g)+O2(g) ⇌ 2N O(g); ΔH = 180kJ −1 A The formation of NO is increased at higher temperature B The volume change at constant pressure does not affect the equilibrium C The pressure change at constant volume does not affect the equilibrium D

Source Image: m.facebook.com
Download Image
Elucidating the Membrane Binding Process of a Disordered Protein: Dynamic Interplay of Anionic Lipids and the Polybasic Region | ACS Physical Chemistry Au Oct 9, 2022When an object is in equilibrium, it means that said object is not accelerating. Since the problem mentions both rotational and translational motion, we need to look at both rotational and translational acceleration. An object can be in equilibrium if a = 0 m/s 2 AND α = 0 rad/s 2. Note that the acceleration says nothing about any velocity the

Source Image: pubs.acs.org
Download Image
Solved Of the following, which is not true of a system at | Chegg.com
Elucidating the Membrane Binding Process of a Disordered Protein: Dynamic Interplay of Anionic Lipids and the Polybasic Region | ACS Physical Chemistry Au Chemistry Question Which of the following conditions is always true at equilibrium? i. ΔG° = ΔG° ^ 1 1 ii. Q = 0 iii. K = 1 iv. ΔG = 0 Solution Answered 2 months ago Create a free account to view solutions By signing up, you accept Quizlet’s Terms of Service and Privacy Policy Continue with Google Continue with Facebook
Solved Which of the following is/are true concerning | Chegg.com Disha Commerce Classes. – 👋Hi Everyone, Lets begin a day with a very positive and motivating quote 🔥 ” Always remember you are a braver than you believe, stronger than you seem Solution Verified by Toppr Correct option is D) solution: option a: It’s an endothermic reaction as ΔH is positive as temperature increases equilibrium will try to balnce it out by moving right side so its true option b : on increasing pressure there is no change in equilibrium as Δn=0 option C :