Oct 2, 2023To identify the unknown isotope X in the following decays: X → 207Pb + e- + ν 7Be + e- → X + ν X → 60Ni + γ Use the following steps: Determine the mass number of X. Determine the atomic number of X. To determine the mass number of X, use the following equation: Mass number of X = Mass number of Y + Mass number of Z
SOLVED: Identify the unknown isotope X in the following decays: 13Be → X + n 230Th → X + X 7207Pb + e- + v 18F → 7X + e- + v
Nov 28, 2022The isotopes ‘X’ in the given nuclear decay sequences can be identified as Thorium-228, Bismuth-207, Lithium-7, and Nickel-60, based on the types (alpha, beta, positron emission, and gamma) of the decays. Explanation: In the provided decay sequences, the isotopes X can be identified as follows: For a.

Source Image: u-of-o-nmr-facility.blogspot.com
Download Image
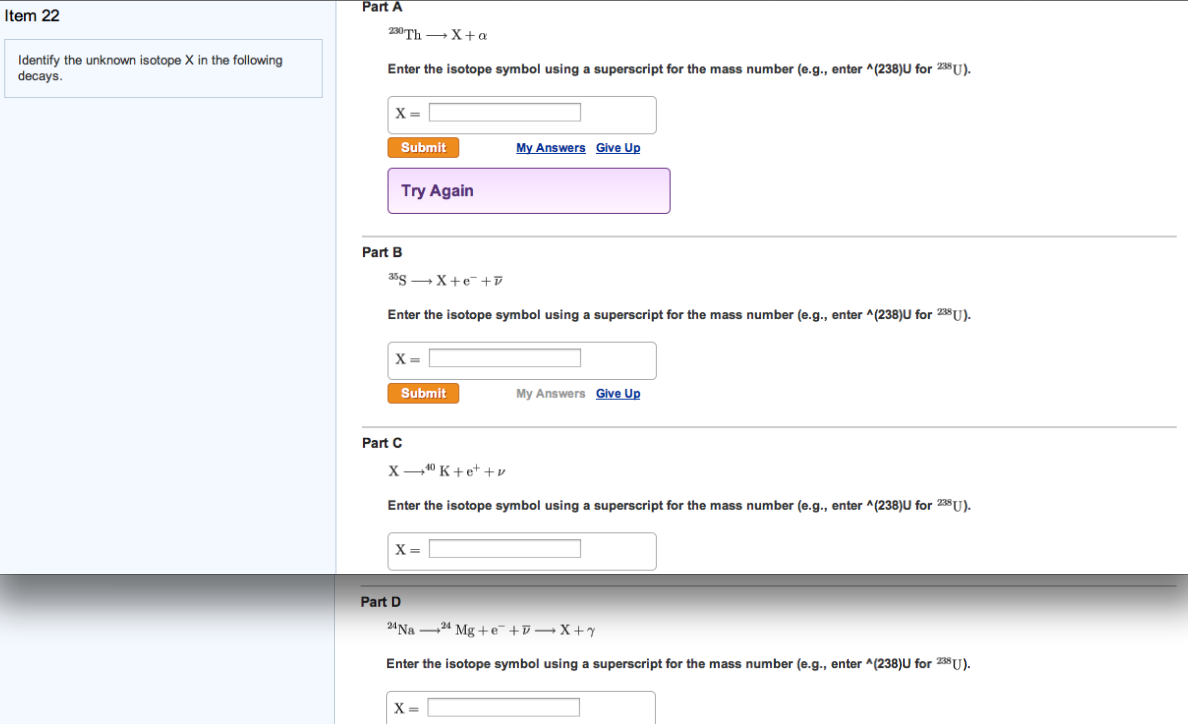
SOLVED: Identify the unknown isotope X in each of the following decays: Enter X in the form Y-A; where Y is the one or two letter chemical symbol and A is the mass number: (a) X-228Ra + α (b) X-19F + e- + v (c) X-239Pu + Y (d) 55Fe + e-X + v Suggested Textbook Physics: Principles with Applications Douglas C. Giancoli 7th Edition
Source Image: chegg.com
Download Image
Radiocarbon (14C) Dating of Qurʾān Manuscripts in: Qurʾān Quotations Preserved on Papyrus Documents, 7th-10th Centuries Identify the unknown isotope X in the following decays. (a) 234U → X + α. (b) 32P → X + e− (c) X → 30Si + e+ (d) 24Mg → X + 5. What is the energy (in MeV) released in the alpha decay of 239Pu? 6. The radioactive hydrogen isotope 3H is called tritium. It decays by beta-minus decay with a half- life of 12.3 years.

Source Image: pubs.acs.org
Download Image
Identify The Unknown Isotope X In The Following Decays.
Identify the unknown isotope X in the following decays. (a) 234U → X + α. (b) 32P → X + e− (c) X → 30Si + e+ (d) 24Mg → X + 5. What is the energy (in MeV) released in the alpha decay of 239Pu? 6. The radioactive hydrogen isotope 3H is called tritium. It decays by beta-minus decay with a half- life of 12.3 years. Identify the unknown isotope X in the following decays.
Absolute Carbon Stable Isotope Ratio in the Vienna Peedee Belemnite Isotope Reference Determined by 1H NMR Spectroscopy | Analytical Chemistry
Identify the unknown isotope X in the following decays. c. ⁷Be + e⁻ → X + v. Skip to main content. Physics. Start typing, then use the up and down arrows to select an option from the list. My Course; … How to Identify the Type of Collision 6m. Intro to Center of Mass 15m. 12. Rotational Kinematics 2h 17m. Worksheet. Oxygen isotopic heterogeneity in the early Solar System inherited from the protosolar molecular cloud | Science Advances

Source Image: science.org
Download Image
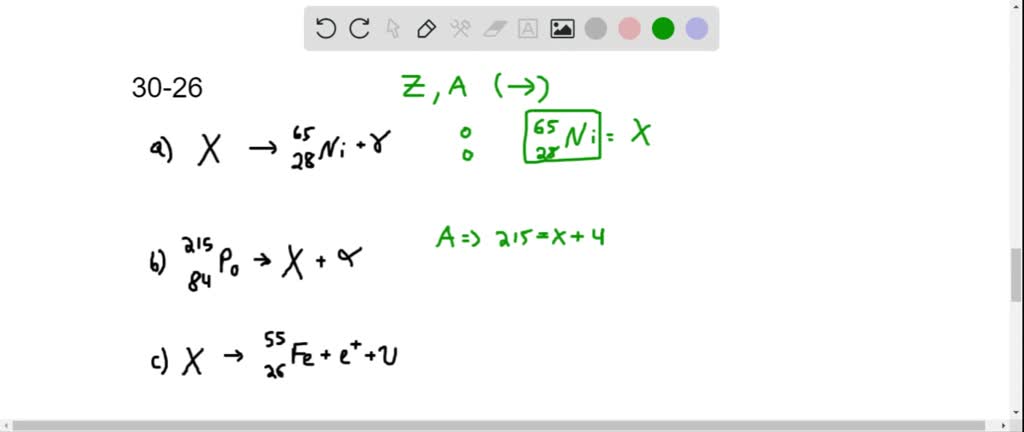
⏩SOLVED:Identify the unknown nuclide or particle (X). (a) X →^6528… | Numerade Identify the unknown isotope X in the following decays. c. ⁷Be + e⁻ → X + v. Skip to main content. Physics. Start typing, then use the up and down arrows to select an option from the list. My Course; … How to Identify the Type of Collision 6m. Intro to Center of Mass 15m. 12. Rotational Kinematics 2h 17m. Worksheet.
Source Image: numerade.com
Download Image
SOLVED: Identify the unknown isotope X in the following decays: 13Be → X + n 230Th → X + X 7207Pb + e- + v 18F → 7X + e- + v Oct 2, 2023To identify the unknown isotope X in the following decays: X → 207Pb + e- + ν 7Be + e- → X + ν X → 60Ni + γ Use the following steps: Determine the mass number of X. Determine the atomic number of X. To determine the mass number of X, use the following equation: Mass number of X = Mass number of Y + Mass number of Z

Source Image: numerade.com
Download Image
Radiocarbon (14C) Dating of Qurʾān Manuscripts in: Qurʾān Quotations Preserved on Papyrus Documents, 7th-10th Centuries SOLVED: Identify the unknown isotope X in each of the following decays: Enter X in the form Y-A; where Y is the one or two letter chemical symbol and A is the mass number: (a) X-228Ra + α (b) X-19F + e- + v (c) X-239Pu + Y (d) 55Fe + e-X + v Suggested Textbook Physics: Principles with Applications Douglas C. Giancoli 7th Edition

Source Image: brill.com
Download Image
kinetics – Determining Half-lifes from a graph – Chemistry Stack Exchange Identify the unknown isotope X in the following decays. a.

Source Image: chemistry.stackexchange.com
Download Image
Part II Particle and Nuclear Physics Examples Sheet – High Energy … Identify the unknown isotope X in the following decays. (a) 234U → X + α. (b) 32P → X + e− (c) X → 30Si + e+ (d) 24Mg → X + 5. What is the energy (in MeV) released in the alpha decay of 239Pu? 6. The radioactive hydrogen isotope 3H is called tritium. It decays by beta-minus decay with a half- life of 12.3 years.

Source Image: yumpu.com
Download Image
Metal Stable Isotope Signatures as Tracers in Environmental Geochemistry | Environmental Science & Technology Identify the unknown isotope X in the following decays.

Source Image: pubs.acs.org
Download Image
⏩SOLVED:Identify the unknown nuclide or particle (X). (a) X →^6528… | Numerade
Metal Stable Isotope Signatures as Tracers in Environmental Geochemistry | Environmental Science & Technology Nov 28, 2022The isotopes ‘X’ in the given nuclear decay sequences can be identified as Thorium-228, Bismuth-207, Lithium-7, and Nickel-60, based on the types (alpha, beta, positron emission, and gamma) of the decays. Explanation: In the provided decay sequences, the isotopes X can be identified as follows: For a.
Radiocarbon (14C) Dating of Qurʾān Manuscripts in: Qurʾān Quotations Preserved on Papyrus Documents, 7th-10th Centuries Part II Particle and Nuclear Physics Examples Sheet – High Energy … Identify the unknown isotope X in the following decays. a.